Abstract
Introduction:
Treatment of adults with relapsed/refractory (R/R) B-ALL using CD19-targeted chimeric antigen receptor (CAR) T cells has achieved remarkable remission rates, both in pediatric and adult populations. There are multiple CAR constructs and T cell manufacturing platforms in use, and both aspects of the therapy may impact efficacy and toxicity. Park et al. report that 83% of adult patients (pts) achieve complete response (CR) to their CD19 CAR T cells with a CD28 costimulatory domain (NEJM; 3785: 449), using an unselected peripheral blood (PBMC) manufacturing platform. Unfortunately, therapy-associated toxicities in adult and pediatric ALL pts are problematic, with grade 3/4 cytokine release syndrome (CRS) ranging from 26-49 % and neurotoxicity 18-42%. Here we report preliminary data from one arm of a phase 1 clinical trial (NCT02146924) in adult pts with R/R B-ALL testing a memory-enriched T cell starting population engineered to express a CD19-specific, CD28-costimulatory CAR (CD19:28z-CAR). All pts achieved CR or CRi with a low incidence of severe cytokine release syndrome (CRS) and neurotoxicity.
Unique to this study is our Tn/mem-enriched manufacturing platform, a naïve/memory T cell-enriched T cell product that is lentivirally transduced to express our CD19:28z-CAR. The manufacturing process starts with patient PBMC, depletes the CD14+ monocytes and CD25+ Tregs, and selects for CD62L+ T cells. The resultant T cell population for CAR transduction includes both the central memory and stem cell memory populations along with naïve T cells. Preclinical studies in mice had suggested that using a more uniform T cell product with a less-differentiated T cell phenotype improved antitumor activity. This Tn/mem manufacturing platform is the same as our Tcm-derived platform (Blood;127:2980) except that CD45RA depletion was omitted.
Patients and Methods:
This phase I study used the activity constrained for toxicity (ACT) design, an extension of the toxicity equivalence range (TEQR) design of Blanchard and Longmate (Contemp Clin Trials; 32:114), that dose escalates based on lack of activity, while constraining the dose for toxicity. The primary objectives of this study were to test the safety and activity of Tn/mem-enriched CD19:28z CAR T cells, and to determine the phase 2 recommended dose. The primary endpoints were toxicity and disease response.
Sixteen pts were consented and received a lymphodepleting regimen (LDR) of 1.5-3 gm/m2 cyclophosphamide over 2-3 days and 25-30 mg/m2 fludarabine for 3 days. Three pts received LDR, but did not receive T cells due to infection or lack of CD19+ disease. Patients received a flat dose of 200 million (M) CD19:28z-CAR T cells: 11 autologous and 2 allogeneic donor products. Of the 13 that received 200 M CAR+ T cells, 2 pts were deemed ineligible for dose escalation / disease response evaluation, as 1 received <80% of the prescribed dose (100 M) and the other had CD19-negative extramedullary disease.
The median age of the 13 CAR T cell treated pts was 33 years (24-72). All pts had active bone marrow (BM) disease at the time of LDR: 8 pts (62%) had high disease burden (15-91% BM blasts) and 5 had low disease burden (</= 5% BM blasts). Patients were heavily pretreated, with a median of 5 (2-6), prior regimens. Six pts received prior allogeneic transplant (HSCT), 9 had prior blinatumomab, and 1 had prior CD19 CAR T cells.
Results:
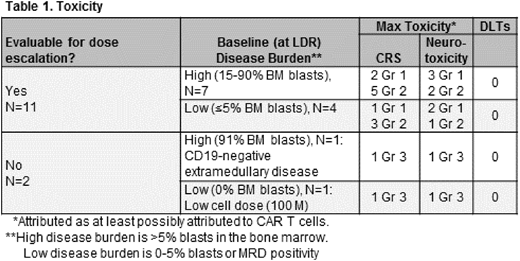
Toxicity: Table 1 describes the major toxicities of the 13 CAR-treated pts, stratified based on disease burden. There were no DLTs, and T-cell therapy attributed (>/=possibly) toxicities were typically mild and reversible. Eight pts had grade 2 CRS, and 2 had grade 3 CRS. Three pts had grade 2 neurotoxicity and 2 had grade 3.
Response: Eleven pts were evaluable for response, with best response of 4 CRs (MRD- by flow) and 7 CRi (6 MRD-, 1 not tested). Median response duration at last contact or HSCT start was 81 days (39-286); 8 pts proceeded to HSCT (in CR or CRi) at a median of 69 days post-CAR infusion (39-103).
Conclusions:
Our ongoing phase 1 trial demonstrates a 100% response rate to Tn/mem-enriched CD19:28z-CAR T cell therapy in adults with relapsed/refractory (R/R) B-ALL. Although the numbers are small, the unanimous response, combined with a tolerable and reversible toxicity profile in pts with both low and high disease burden is remarkable and suggests promise for this Tn/mem manufacturing platform for CD19 and other CAR targets.
Khaled:Juno: Other: Travel Funding; Daiichi: Consultancy; Alexion: Consultancy, Speakers Bureau. Wang:Mustang Therapeutics: Other: Licensing Agreement, Patents & Royalties, Research Funding. Brown:Mustang Therapeutics: Consultancy, Other: Licensing Agreement, Patents & Royalties, Research Funding. Forman:Mustang Therapeutics: Other: Licensing Agreement, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal